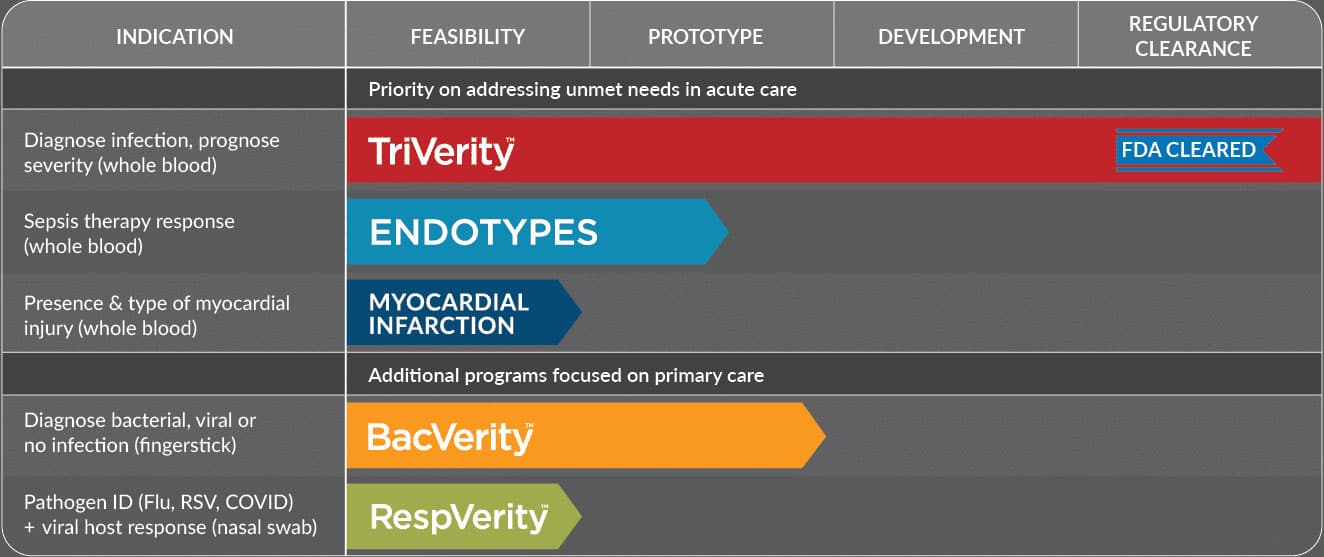
Groundbreaking tests to address unmet needs

We think the precision medicine playbook that has worked in cancer can work in sepsis
Considering that not a single immunomodulatory therapy for sepsis has been successfully brought to market despite 40+ years of trials,1 a novel approach is needed.
A modern understanding of sepsis suggests that the sepsis syndrome may actually be composed of several subtypes of patients. Similar to how breast cancer is now properly defined as composed of different subtypes (e.g., ER/PR/HER2 status), a sepsis patient may someday be properly defined as suffering from overramped innate immunity, adaptive immune collapse, endothelial dysfunction, etc. And just like different breast cancer survival rates have improved as each subtype guides a specific treatment, so may sepsis outcomes improve when specific therapies are paired with individual subtypes.2 Our products are in development, are not for sale, and do not have marketing approval or clearance from regulatory authorities in any jurisdiction.
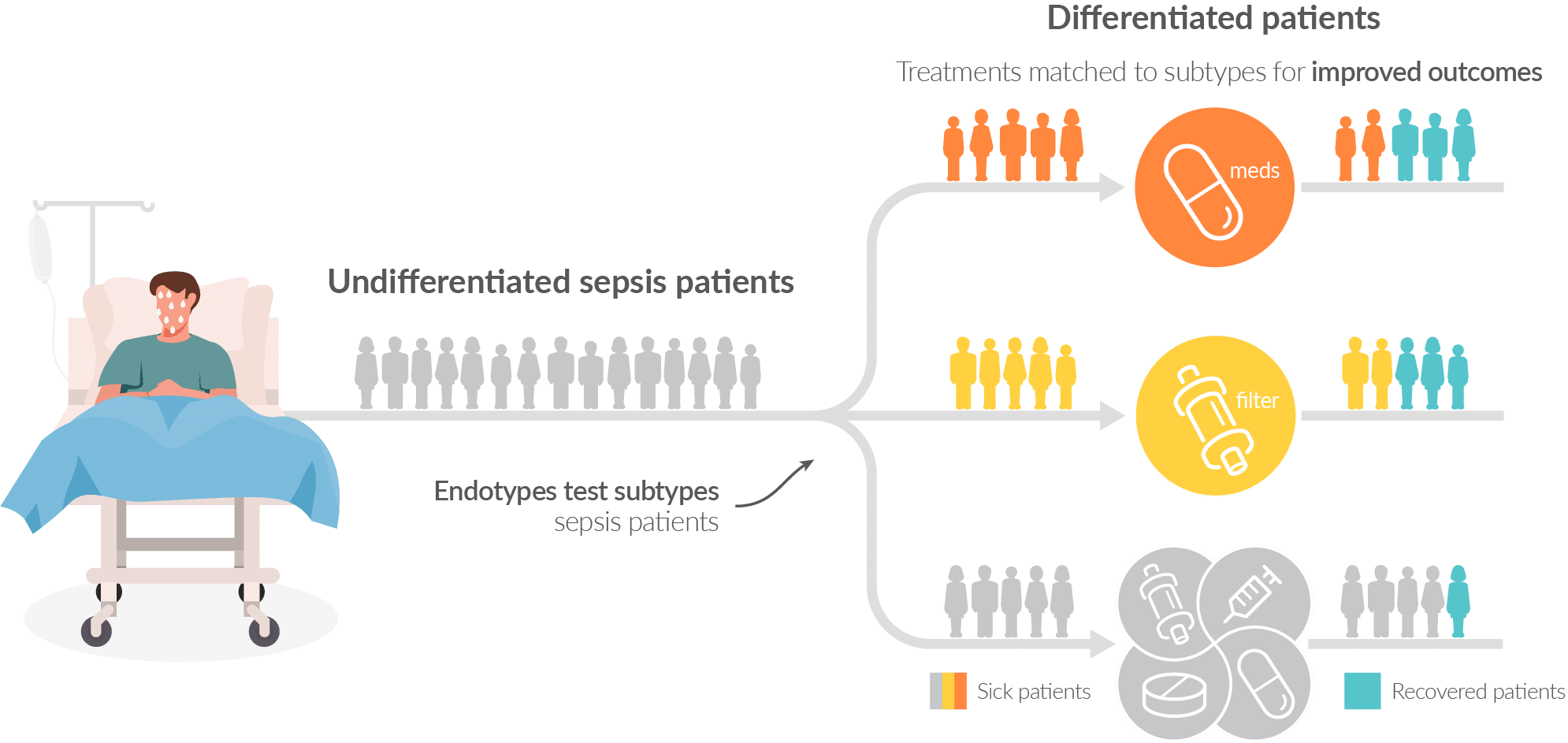
Our Endotypes program is at the forefront in sepsis therapy selection research
Inflammatix has been at the forefront of sepsis subtypes (or ‘endotypes’) research in transcriptomics, with a classifier that has been validated3,4 in research settings to produce subgroups with different outcomes. We are actively working to determine which therapies may show a difference in treatment effects among them.
Inflammatix collaborates with others committed to applying precision medicine in sepsis
Connect with us to partner
Inflammatix is working with drug and device companies to help identify patients likely to respond to novel and existing therapies. Interested parties can contact us at [email protected].

(SUBtyping in SePsis And Critical illnEss)
Inflammatix is also currently sponsoring the SUBSPACE Consortium, a collaborative of leading researchers and clinicians comparing different existing subtype classifiers.
To learn more please visit the SUBSPACE Consortium

Inflammatix has several clinical studies in progress. Clinical trialists interested in validating Inflammatix endotypes should contact [email protected].
Looking for other ways to collaborate?
Reach out- Opal S.M., et al., The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C?*. Crit Care Med. 2014 Jul;42(7):1714-21.
- Maslove D.M., et al., Redefining critical illness. Nat Med 28, 1141–1148 (2022).
- Iglesias J., et al., A 33-mRNA Classifier Is Able to Produce Inflammopathic, Adaptive, and Coagulopathic Endotypes with Prognostic Significance: The Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis (ORANGES) Trial. J. Pers. Med. 2021; 11(1):9.
- Sweeney T.E., et al., Validation of Inflammopathic, Adaptive, and Coagulopathic Sepsis Endotypes in Coronavirus Disease 2019. Crit Care Med: February 2021 – Volume 49 – Issue 2 – p e170-e178.1; 51:e13626.