INFLAMMATIX PIPELINE
Groundbreaking tests to address unmet needs
Inflammatix host response diagnostics are powered by ‘reading’ the immune system. Our immune system has developed targeted responses to different types of infections and other diseases over millions of years of evolution.
We are harnessing the ability to interpret the immune response to develop tests aimed to help physicians make confident and timely clinical decisions.
Our products are in development, are not for sale, and do not have marketing approval or clearance from regulatory authorities in any jurisdiction.

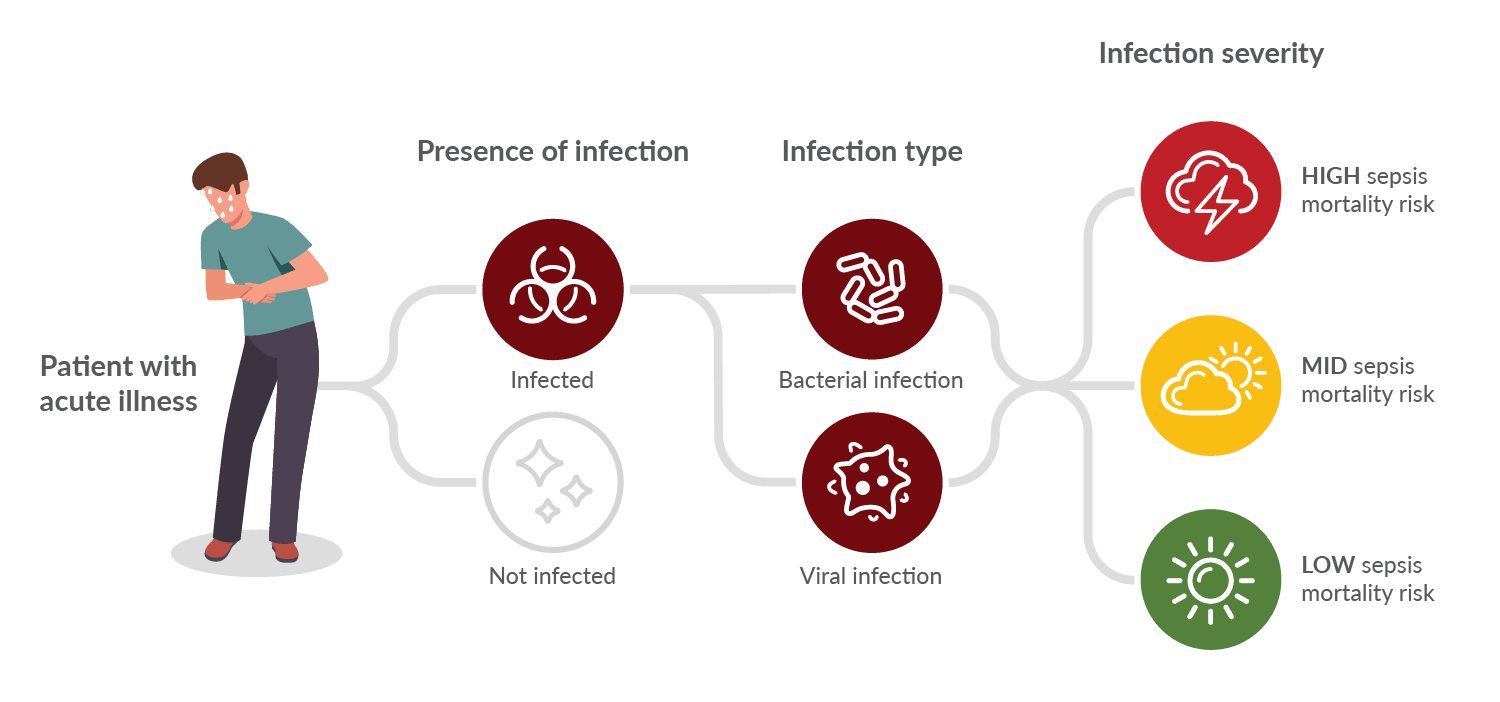
Acute Infection and Sepsis Test
Shooting for the holy grail: Identify the presence, type and severity of an acute infection.
The test is being developed for emergency rooms, urgent care clinics and inpatient use to diagnose (or rule out) acute infection and sepsis in multiple clinical scenarios:
- First presentation in the ED or urgent care clinic
- Identifying infections in traumatic injuries
- ICU patient monitoring
Product in development, is not for sale, and does not have marketing approval or clearance from regulatory authorities in any jurisdiction.


Acute Infection Test
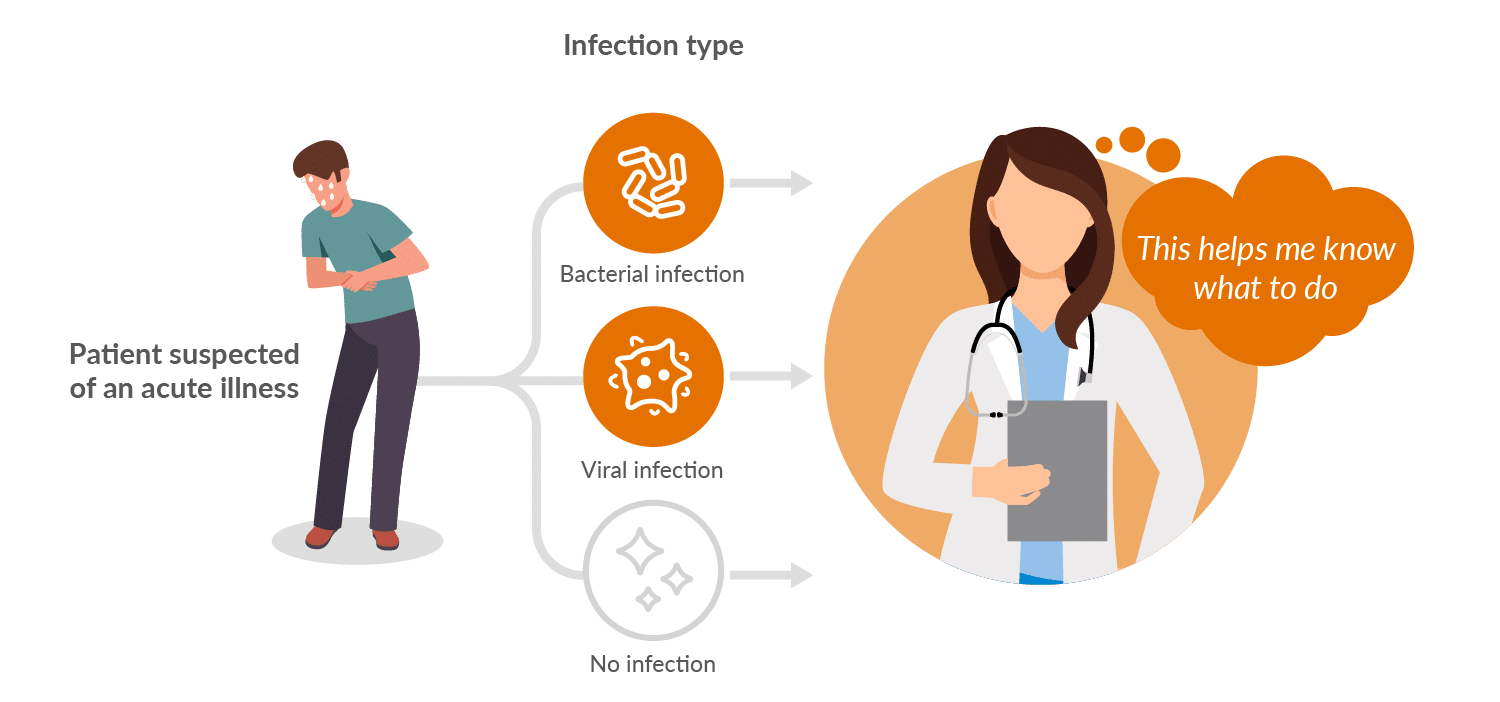
Soon, we’ll answer your simple question:
Is my infection bacterial or viral?
Ideal for outpatient clinics and urgent care centers. Safely and confidently avoid prescribing antibiotics on negative results (development work suggests the BacVerity test can achieve a greater than 95% negative predictive value for bacterial infection) [1]
Product in development, is not for sale, and does not have marketing approval or clearance from regulatory authorities in any jurisdiction.


Therapy Response Prediction
Unlocking personalized therapies in sepsis and critical illness
Sepsis is defined as a ‘life-threatening dysregulated host response to infection’, and for years, clinical trials have tried and failed to find immunomodulatory therapies for sepsis to bring homeostasis to the immune system. In fact, not a single immunomodulatory therapy for sepsis has been successfully brought to market despite 40+ years of trials [1].
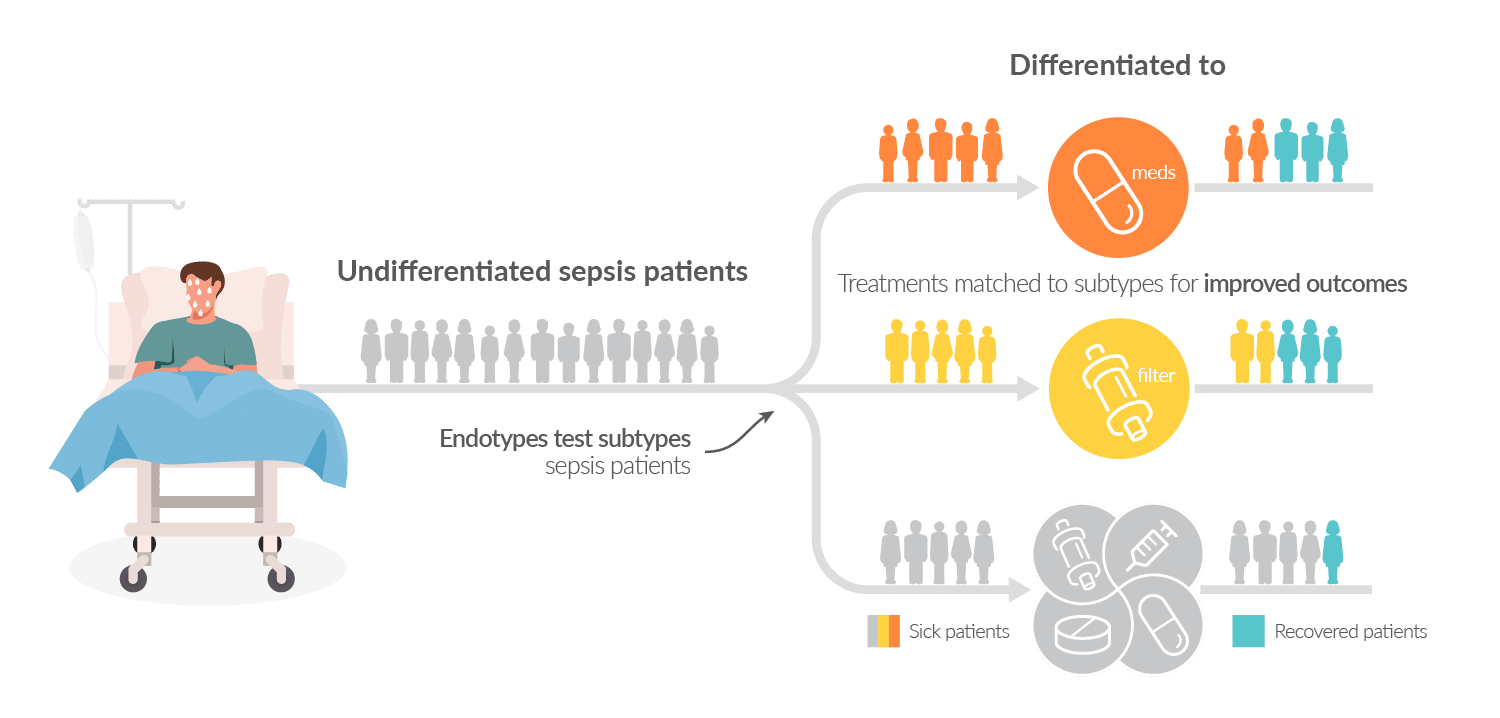
A modern understanding of sepsis using new kinds of molecular and clinical data suggests that the sepsis syndrome may actually be composed of several subtypes of patients. Just like how breast cancer is now properly defined as composed of different subtypes (e.g. ER/PR/HER2 status), a sepsis patient may someday be properly defined as suffering from overramped innate immunity, adaptive immune collapse, endothelial dysfunction, etc. And just like different breast cancer survival rates have improved as each subtype finds a specific treatment, so may sepsis outcomes improve when specific therapies are paired with individual subtypes [2].
Inflammatix has been at the forefront of sepsis subtypes (or ‘endotypes’) research in transcriptomics, with a 33-mRNA classifier that has been repeatedly validated [3, 4] in research settings to produce three subgroups with different outcomes. We are actively working to determine which therapies may show a difference of treatment effect among the three subtypes: 1-Inflammapathic, 2-Adaptive, 3-Coagulopathic.
Product in development, is not for sale, and does not have marketing approval or clearance from regulatory authorities in any jurisdiction.
- Opal SM, Dellinger RP, Vincent JL, Masur H, Angus DC. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C?*. Crit Care Med. 2014 Jul;42(7):1714-21.
- Maslove DM, Tang B, Shankar-Hari M, et al. Redefining critical illness. Nat Med 28, 1141–1148 (2022).
- Iglesias J, Vassallo AV, Liesenfeld O, Levine JS, Patel VV, Sullivan JB, Cavanaugh JB, Elbaga Y, Sweeney TE. A 33-mRNA Classifier Is Able to Produce Inflammopathic, Adaptive, and Coagulopathic Endotypes with Prognostic Significance: The Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis (ORANGES) Trial. Journal of Personalized Medicine. 2021; 11(1):9.
- Sweeney TE, Liesenfeld O, Wacker J, He Y, et al. Validation of Inflammopathic, Adaptive, and Coagulopathic Sepsis Endotypes in Coronavirus Disease 2019. Critical Care Medicine: February 2021 – Volume 49 – Issue 2 – p e170-e178.
Our Approach
Our approach to developing host response-based diagnostic tests has yielded remarkable results to date
Focus on what clinicians want
Every test should be built with a physician end-user in mind. Our team is physician-led, ensuring that development starts with the question of whether a test could be clinically actionable, and whether it fits into workflow. Our test for sepsis was built to answer a critical clinical gap: physicians usually guess about whether or not a patient needs antibiotics. Current pathogen-focused diagnostic methods fail to answer this question because they frequently miss infections that haven’t spread to the bloodstream. Thus, we focused on using the immune response to infections to answer key questions in the clinical evaluation of an acutely ill patient: (1) does the patient need antibiotics, and (2) what level of care is required? These questions are best answered by ‘reading’ the immune response, rather than looking directly for a pathogen as a ‘needle in a haystack’.
Embrace heterogenous data in product development
Embrace heterogenous data in selecting and analyzing cohorts to study. Acute infections can be remarkably clinically diverse: kids and adults, inpatients and outpatients, in different settings around the world. We wanted to build a test that would work in every environment. We thus embrace this clinical heterogeneity by analyzing multiple cohorts that are diverse in their population, sample types, assays used and other factors. Our robust statistical pipeline allows us to find reproducible signal in the ‘noise’ of multiple datasets. Although this is a challenging approach, our discovery methods ensure that the diagnostics we find are generalizable to new populations.
Validate externally
Validate on multiple, independent and diverse cohorts. Trustworthy tests must demonstrate robust performance in independent, blinded multi-center studies.
Deep Pipeline of Novel, Rapid Tests
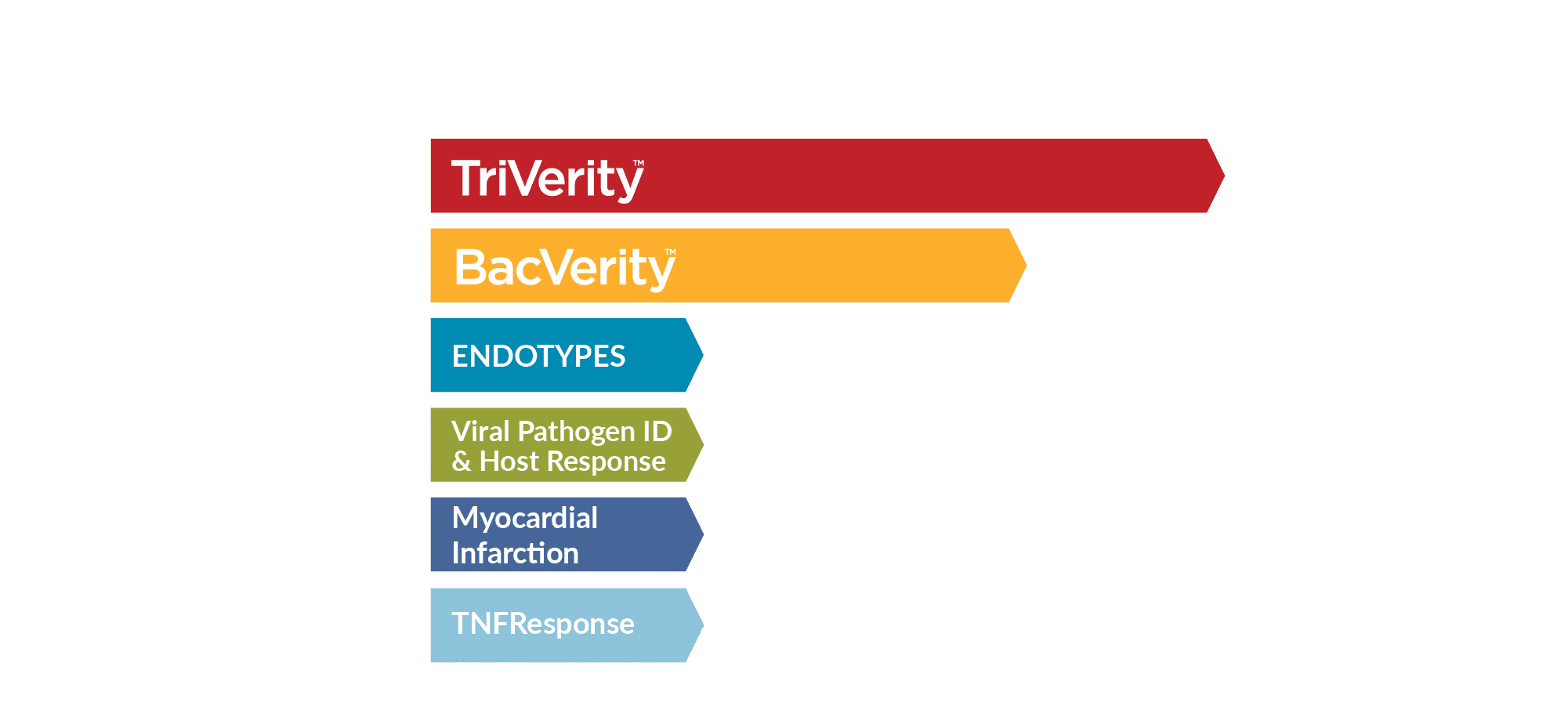
Products in development, are not for sale, and do not have marketing approval or clearance from regulatory authorities in any jurisdiction.



